Reversed phase analysis of antibody fragments
Reversed phase analysis of antibody fragments
TOSOH
Application n°a15l46a
Introduction
Antibody therapeutics are enjoying high growth rates in the biopharmaceuticals market, the major therapeutic areas being cancer and immune/inflammation related disorders including arthritis and multiple sclerosis. The characterisation of these biomolecules is a major challenge in process monitoring and quality control. The main product characteristics to be monitored are aggregate and fragment content, glycosylation pattern and charged isoforms. Besides SEC and IEC, Reversed phase chromatography (RPC) can be applied for the analysis of proteins and protein fragments. A new C4 reversed phase column for protein analysis was applied to separate heavy and light chain and Fc and Fab fragments of selected monoclonal antibodies.
RPC columns for protein analysis
Reversed phase chromatography is one of the most frequently used chromatographic modes for analytical separations. It is most often used for the analysis of small molecular weight compounds, but there exist various standard applications for the separation of biomolecules, such as proteins. Conventional HPLC packing materials with 8-14 nm pore sizes are not generally suitable for the analysis of large intact proteins, as the analytes are not able to access the surface area within these pores. Silica based TSKgel TMS-250 with 25 nm pores and polymer based TSKgel Octadecyl-4PW (50 nm) and Phenyl-5PW (100 nm) have been widely applied for the reversed phase separation of intact proteins. Now a new silica based wide pore butyl/C4 phase, the TSKgel Protein C4-300, completes the existing range of TSKgel RPC protein columns.
TSKgel Protein C4-300 columns are available in 2 and 4.6 millimeter inner diameter and 5, 10 and 15 cm column length. The pore size of 30 nm is ideal for the separation of proteins. A particle size of 3 µm and the optimized ligand density and alkyl length result in better protein and peptide resolution compared to other leading RP-C4 HPLC phases. Latest surface modification techniques and TMS endcapping reduce undesirable secondary interactions and peak tailing.
Separation of standard proteins
Figure 1 shows the separation of a mixture of standard proteins on the new C4 column compared to c competitor column with 3.5 µm particle size. The resolution between cytochrome c and lysozymes reaches 24.8 on the TSKgel C4 column compared to 18.6 on the competitor C4 column. Further, the TSKgel column shows higher theoretical plates and less peak tailing, especially for BSA (Peak 4) and also a better resolution of minor peaks. For high speed separations the analysis time can be reduced by more than 80% when using the 5 cm long column and increasing the flow rate to 3 mL/min (Figure 2). The backpressure remains below 15 MPa allowing the use of standard HPLC systems.
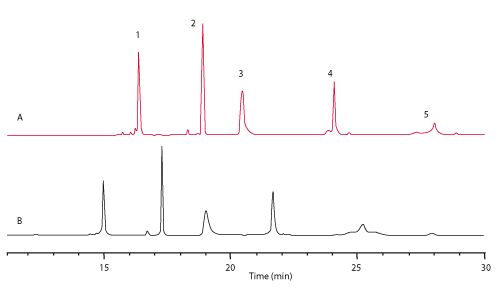
FIGURE 1_Column A: TSKgel Protein C4-300 3 µm (4.6 mm ID x 15 cm L);
B: Competitor C4 3.5 µm (4.6 mm ID x 15 cm L);
Sample: [1] cytochrome c, [2] lysozyme, [3] BSA, [4] a-chymotrypsinogen A, [5] ovalbumin;
Mobile phase: A: H2O/ACN/TFA=90/10/0.05 (v/v/v); B: H2O/ACN/TFA=20/80/0.05 (v/v/v);
Gradient: 0% - 100% B in 45 min; Flow rate: 1 mL/min; Temp.: 40°C;
Detection: UV @ 210 nm
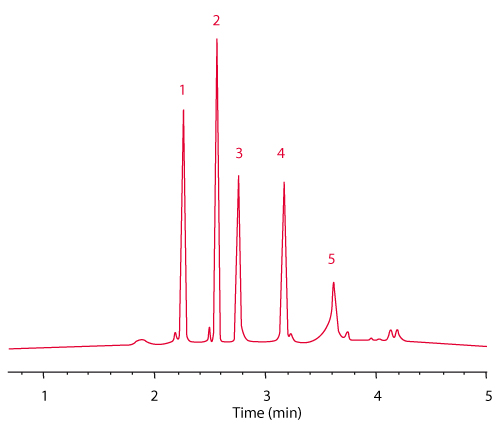
FIGURE 2_Column A: TSKgel Protein C4-300 3 µm (4.6 mm ID x 5 cm L);
Sample [1] cytochrome c, [2] lysozyme, [3] BSA, [4] a-chymotrypsinogen A, [5] ovalbumin;
Mobile phase: A: H2O/ACN/TFA=90/10/0.05 (v/v/v);
B: H2O/ACN/TFA=20/80/0.05 (v/v/v);
Gradient: 0% - 100% B in 5 min;
Flow rate: 3 mL/min;
Temp.: 40°C; Detection: UV @ 210 nm
Analysis of antibody fragments
TSKgel Protein C4-300 is ideally suited for the analysis of peptides, protein fragments, and intact proteins, such as antibodies, recombinant proteins, or PEGylated proteins. Figure 3 shows the separation of Fab’, Fc and F(ab’)2 antibody fragments on a standard column with a linear gradient of water/acetonitrile/TFA from 90 % water to 80 % acetonitrile.
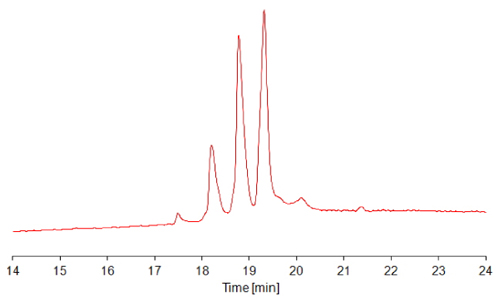
FIGURE 3_Column: TSKgel Protein C4-300, 4.6 mm ID x 15 cm L;
Mobile phase A: H2O/ACN/TFA = 90/10/0.05 (v/v/v);
Mobile phase B: H2O/ACN/TFA = 20/80/0.05 (v/v/v);
Gradient: 0% - 100% B in 45 min;
Temperature: 40°C;
Detection: UV @ 210 nm;
Inj. vol.: 10 μL;
Samples: papain-digested IgG
Figure 4 shows the analysis of heavy and light chains of twoantibodies, IgG-A and IgG-B. The samples were reduced with dithiothreitol to dissociate into heavy chain and light chain,and then applied to the TSKgel Protein C4-300 RPC column. The chromatograms show small differences in hydrophobicity between IgG-A and IgG-B. In addition, the chains of IgG-B are eluted as broader peak with shoulder, indicating IgG-B is heterogeneous, and a hydrophilic variant is present.
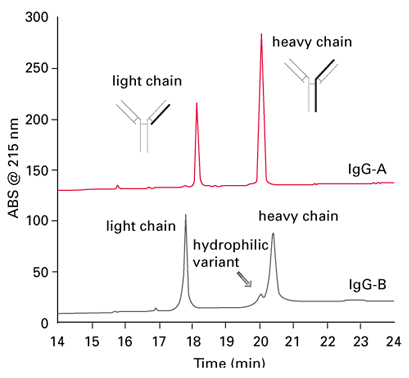
FIGURE 4_Column: TSKgel Protein C4-300, 4.6 mm ID x 15 cm L;
Mobile phase A: H2O/ACN/TFA = 90/10/0.05 (v/v/v);
Mobile phase B: H2O/ACN/TFA = 20/80/0.05 (v/v/v);
Gradient: 0% - 100% B in 45 min; Temperature: 50°C;
Detection: UV @ 215 nm; Inj. vol.: 100 µL;
Samples: [1] IgG-A (mouse monoclonal), [2] IgG-B (mouse monoclonal),
each reduced with dithiothreitol

TOSOH
Application n°a15l46a
Introduction
Antibody therapeutics are enjoying high growth rates in the biopharmaceuticals market, the major therapeutic areas being cancer and immune/inflammation related disorders including arthritis and multiple sclerosis. The characterisation of these biomolecules is a major challenge in process monitoring and quality control. The main product characteristics to be monitored are aggregate and fragment content, glycosylation pattern and charged isoforms. Besides SEC and IEC, Reversed phase chromatography (RPC) can be applied for the analysis of proteins and protein fragments. A new C4 reversed phase column for protein analysis was applied to separate heavy and light chain and Fc and Fab fragments of selected monoclonal antibodies.
RPC columns for protein analysis
Reversed phase chromatography is one of the most frequently used chromatographic modes for analytical separations. It is most often used for the analysis of small molecular weight compounds, but there exist various standard applications for the separation of biomolecules, such as proteins. Conventional HPLC packing materials with 8-14 nm pore sizes are not generally suitable for the analysis of large intact proteins, as the analytes are not able to access the surface area within these pores. Silica based TSKgel TMS-250 with 25 nm pores and polymer based TSKgel Octadecyl-4PW (50 nm) and Phenyl-5PW (100 nm) have been widely applied for the reversed phase separation of intact proteins. Now a new silica based wide pore butyl/C4 phase, the TSKgel Protein C4-300, completes the existing range of TSKgel RPC protein columns.
TSKgel Protein C4-300 columns are available in 2 and 4.6 millimeter inner diameter and 5, 10 and 15 cm column length. The pore size of 30 nm is ideal for the separation of proteins. A particle size of 3 µm and the optimized ligand density and alkyl length result in better protein and peptide resolution compared to other leading RP-C4 HPLC phases. Latest surface modification techniques and TMS endcapping reduce undesirable secondary interactions and peak tailing.
Separation of standard proteins
Figure 1 shows the separation of a mixture of standard proteins on the new C4 column compared to c competitor column with 3.5 µm particle size. The resolution between cytochrome c and lysozymes reaches 24.8 on the TSKgel C4 column compared to 18.6 on the competitor C4 column. Further, the TSKgel column shows higher theoretical plates and less peak tailing, especially for BSA (Peak 4) and also a better resolution of minor peaks. For high speed separations the analysis time can be reduced by more than 80% when using the 5 cm long column and increasing the flow rate to 3 mL/min (Figure 2). The backpressure remains below 15 MPa allowing the use of standard HPLC systems.

FIGURE 1_Column A: TSKgel Protein C4-300 3 µm (4.6 mm ID x 15 cm L);
B: Competitor C4 3.5 µm (4.6 mm ID x 15 cm L);
Sample: [1] cytochrome c, [2] lysozyme, [3] BSA, [4] a-chymotrypsinogen A, [5] ovalbumin;
Mobile phase: A: H2O/ACN/TFA=90/10/0.05 (v/v/v); B: H2O/ACN/TFA=20/80/0.05 (v/v/v);
Gradient: 0% - 100% B in 45 min; Flow rate: 1 mL/min; Temp.: 40°C;
Detection: UV @ 210 nm

FIGURE 2_Column A: TSKgel Protein C4-300 3 µm (4.6 mm ID x 5 cm L);
Sample [1] cytochrome c, [2] lysozyme, [3] BSA, [4] a-chymotrypsinogen A, [5] ovalbumin;
Mobile phase: A: H2O/ACN/TFA=90/10/0.05 (v/v/v);
B: H2O/ACN/TFA=20/80/0.05 (v/v/v);
Gradient: 0% - 100% B in 5 min;
Flow rate: 3 mL/min;
Temp.: 40°C; Detection: UV @ 210 nm
Analysis of antibody fragments
TSKgel Protein C4-300 is ideally suited for the analysis of peptides, protein fragments, and intact proteins, such as antibodies, recombinant proteins, or PEGylated proteins. Figure 3 shows the separation of Fab’, Fc and F(ab’)2 antibody fragments on a standard column with a linear gradient of water/acetonitrile/TFA from 90 % water to 80 % acetonitrile.

FIGURE 3_Column: TSKgel Protein C4-300, 4.6 mm ID x 15 cm L;
Mobile phase A: H2O/ACN/TFA = 90/10/0.05 (v/v/v);
Mobile phase B: H2O/ACN/TFA = 20/80/0.05 (v/v/v);
Gradient: 0% - 100% B in 45 min;
Temperature: 40°C;
Detection: UV @ 210 nm;
Inj. vol.: 10 μL;
Samples: papain-digested IgG
Figure 4 shows the analysis of heavy and light chains of twoantibodies, IgG-A and IgG-B. The samples were reduced with dithiothreitol to dissociate into heavy chain and light chain,and then applied to the TSKgel Protein C4-300 RPC column. The chromatograms show small differences in hydrophobicity between IgG-A and IgG-B. In addition, the chains of IgG-B are eluted as broader peak with shoulder, indicating IgG-B is heterogeneous, and a hydrophilic variant is present.

FIGURE 4_Column: TSKgel Protein C4-300, 4.6 mm ID x 15 cm L;
Mobile phase A: H2O/ACN/TFA = 90/10/0.05 (v/v/v);
Mobile phase B: H2O/ACN/TFA = 20/80/0.05 (v/v/v);
Gradient: 0% - 100% B in 45 min; Temperature: 50°C;
Detection: UV @ 215 nm; Inj. vol.: 100 µL;
Samples: [1] IgG-A (mouse monoclonal), [2] IgG-B (mouse monoclonal),
each reduced with dithiothreitol
