Characterization Studies of PEGylated Lysozyme
Characterization Studies of PEGylated Lysozyme
TOSOH
Application n°a15l67a
Abstract
PEGylation, the process by which polyethylene glycol (PEG) chains are attached to protein and peptide drugs is a common practice in the development of biopharmaceuticals to prolong serum half-life and improve pharmacokinetics of a drug. There is increasing demand for chromatographic methods to separate the modified isoforms from the native protein. This application note describes the use of size exclusion and ion exchange chromatography for the characterization of PEGylated lysozyme.
INTRODUCTION
Chemical modification of therapeutic proteins in order to enhance their biological activity is of increasing interest. One of the most frequently used protein modification method is the covalent attachment of poly (ethylene glycol) which is called PEGylation.This polymeric modification changes the biochemical and physicochemical properties of the protein, which decreases the in vivo clearance rate and reduces toxicity and immunogenicity of therapeutic proteins. After PEGylation the reaction mixture has to be purified in order to remove non-reacted protein and undesired reaction products. Chromatography as the most common purification method is influenced by PEGylation because of masking and shield effects of the covalently linked PEG molecule.
Lysozyme is a well known standard protein, which is often used to determine the dynamic binding capacity of Ion Exchange Chromatography (IEC) resins; therefore we decided to use PEG-lysozyme as a model protein in our study.
PEGylated lysozyme was produced out of methoxy-PEGaldehyde (with a MW of 5 kDa, 10 kDa and 30 kDa) and chicken egg white lysozyme in phosphate buffer in presence of sodium-cyano-borohydrid (NaCNBH3) as reducing agent. The PEGylation reaction takes place between the aldehyde group of methoxy-PEG-aldehyde and free amino acid group (NH2-group) of lysine residues within the lysozyme (Fig. 1).
The product mixture was analysed by a TSKgel G3000SWXL SEC HPLC-column, SDS-PAGE (not shown), IEC (TSKgel SP-5PW (20) and TSKgel SP-NPR strong cation exchange (SCX)) and subsequent MALDI-TOF MS analysis (not shown).
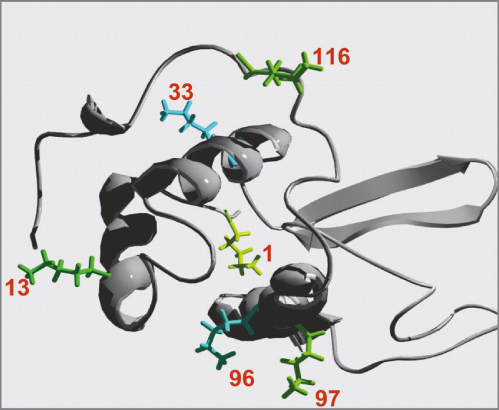
FIGURE 1_Lysozyme has six lysine residues as possible PEGylation reaction sides
METHODS
PEGylation of egg white lysozyme: 5, 10, 30 kDa methoxy PEG-aldehyde; 100 mM Phosphate buffer (Na2HPO4, NaH2PO4) pH 6.0; PEGylation by reductive alkylation; 20 mM NaCNBH3 to reduce a Schiff base; 100 mM HCl to stop PEGylation reaction
SEC-HPLC:
Column: TSKgel G3000SWXL (7.8 mm ID x 30 cm, 5 µm, 250 Å)
HPLC-System: Shimadzu Prominence
Flow rate: 1.0 mL/min
Mobile phase: 0.1 M Phosphate buffer 0.1 M Na2SO4, pH 6.7
Detector: UV 280 nm
Injection vol.: 20 µL
IEC-FPLC:
Column: TSKgel SP-5PW (20) (6.6 mm ID x 22 cm L, 20 µm, 1000 Å)
Flow rate: 0.85 mL/min
Buffer A: 25 mM Phosphate buffer 0.1 M Na2SO4, pH 6.0
Buffer B: A + 0.5 M NaCl
Detector: UV 280 nm
Injection vol.: 100 µL
IEC-HPLC:
Column: TSKgel SP-NPR (4.6 mm ID x 3.5 cm, 2.5 µm)
Flow rate: 1.0 mL/min
Buffer A: 25 mM Phosphate buffer 0.1 M Na2SO4, pH 6.0
Buffer B: A + 0.5 M NaCl
Detector: UV 280 nm
Injection vol.: 5 µL
RESULTS
PEGylation of lysozyme
Figure 2 shows typical chromatogram pattern of a reaction mixture of PEGylated lysozyme separated on TSKgel SP-5PW. PEG chain lengths of 5kDa and 30kDa are shown from the left to right. The profiles indicate a similar reaction characteristic. Non-reacted lysozyme remained in the reaction mixture; mono-PEGylated lysozyme as well as polyPEGylated lysozyme was formed during the reaction.
SEC was performed as shown in Figure 3. By the use of retention volumes from SEC analysis the viscosity radius of PEGylated was SEC-HPLC analysis of reaction mixes determined under assumption of being a globular protein.
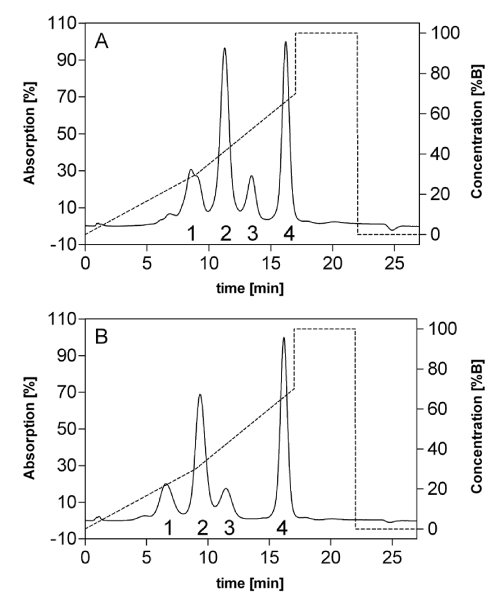
FIGURE 2_Separation of PEGylated lysozymes on an analytical TSKgel SP-5PW SCX column. Peaks were identifying by MALDI-TOF analysis, identical sizes were numbered consecutively

FIGURE 3_SEC analysis of reaction mixtures performed with a TSKgel G3000SWXL column. Lysozyme and PEGylated Lysozyme derivates for all tested sizes are shown.
Selectivity
The particle size was of great importance for the selectivity. Especially the non-porous particle resin of the prepacked TSKgel SP-NPR column showed a very high resolution; with number of mono-PEGylated isoforms while two isoforms were visible for di-PEGylated lysozyme. TSKgel SP-5PW (20) is polishing resin with a particle size ten times bigger than the SP-NPR matrix. The resolution decreased, but two mono-PEGylated isoforms still remained visible (Fig. 4 A and B).
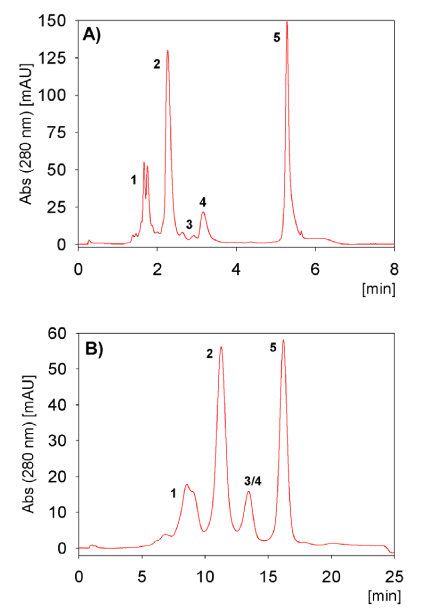
FIGURE 4_Resolution dependency on particle size shown with 5kDa PEGylated lysozyme reaction mixture. (A) TSKgel SP-NPR, (B) TSKgel SP-5PW; (1) poly-PEG5Lys, (2) 1-mono- PEG5Lys, (3) 2-mono- PEG5Lys, (4) 3-monoPEG5Lys and (5) lysozyme
DISCUSSION
Lysozyme as model protein was PEGylated to examine the behaviour of PEGylated proteins in cation exchange chromatography. A random PEGylation of lysozyme using methoxy-PEG-aldehyde of sizes 5kDa, 10 kDa and 30 kDa was performed.
In Size Exclusion Chromatography a massive increase of size by PEGylation was observed. The SEC elution behaviour of lysozyme modified with a 30 kDa PEG was equal to a 450 kDa globular protein. There was a linear correlation between the theoretical MW of PEGylated protein and the MW calculated via SEC. This result illustrates the influence of PEG on the hydrodynamic radius of PEGylated protein.
Selectivity comparison
Cation exchange chromatography was capable to resolve the PEGylated isomers which are product of the random PEGylation. The use of non-porous SP-NPR polishing resin leads to the best resolution. This is due to the better mass transfer kinetics for large molecules on small, non porous particles
Despite the loss in resolution it was useful to use a porous resin with larger particle size for the first chromatographic step because of higher capacity and better pressure-flowcharacteristics.
CONCLUSION
The selectivity of various cation exchanger resins were evaluated with random PEGylated lysozyme (chicken egg white). It is shown that the selectivity for PEG modified proteins depends on particle size of the resin. All PEGylated lysozyme species could be resolved on a TSKgel SP-NPR column with a particle size of 2.5 µm and on a TSKgel SP-5PW column with a particle size of 20 µm. A further increase of particle size leads to loss of resolution.
REFERENCE
A. Moosmann et al. J. Chromatogr. A (2010) 1217 (2): P.209-215.

TOSOH
Application n°a15l67a
Abstract
PEGylation, the process by which polyethylene glycol (PEG) chains are attached to protein and peptide drugs is a common practice in the development of biopharmaceuticals to prolong serum half-life and improve pharmacokinetics of a drug. There is increasing demand for chromatographic methods to separate the modified isoforms from the native protein. This application note describes the use of size exclusion and ion exchange chromatography for the characterization of PEGylated lysozyme.
INTRODUCTION
Chemical modification of therapeutic proteins in order to enhance their biological activity is of increasing interest. One of the most frequently used protein modification method is the covalent attachment of poly (ethylene glycol) which is called PEGylation.This polymeric modification changes the biochemical and physicochemical properties of the protein, which decreases the in vivo clearance rate and reduces toxicity and immunogenicity of therapeutic proteins. After PEGylation the reaction mixture has to be purified in order to remove non-reacted protein and undesired reaction products. Chromatography as the most common purification method is influenced by PEGylation because of masking and shield effects of the covalently linked PEG molecule.
Lysozyme is a well known standard protein, which is often used to determine the dynamic binding capacity of Ion Exchange Chromatography (IEC) resins; therefore we decided to use PEG-lysozyme as a model protein in our study.
PEGylated lysozyme was produced out of methoxy-PEGaldehyde (with a MW of 5 kDa, 10 kDa and 30 kDa) and chicken egg white lysozyme in phosphate buffer in presence of sodium-cyano-borohydrid (NaCNBH3) as reducing agent. The PEGylation reaction takes place between the aldehyde group of methoxy-PEG-aldehyde and free amino acid group (NH2-group) of lysine residues within the lysozyme (Fig. 1).
The product mixture was analysed by a TSKgel G3000SWXL SEC HPLC-column, SDS-PAGE (not shown), IEC (TSKgel SP-5PW (20) and TSKgel SP-NPR strong cation exchange (SCX)) and subsequent MALDI-TOF MS analysis (not shown).

FIGURE 1_Lysozyme has six lysine residues as possible PEGylation reaction sides
METHODS
PEGylation of egg white lysozyme: 5, 10, 30 kDa methoxy PEG-aldehyde; 100 mM Phosphate buffer (Na2HPO4, NaH2PO4) pH 6.0; PEGylation by reductive alkylation; 20 mM NaCNBH3 to reduce a Schiff base; 100 mM HCl to stop PEGylation reaction
SEC-HPLC:
Column: TSKgel G3000SWXL (7.8 mm ID x 30 cm, 5 µm, 250 Å)
HPLC-System: Shimadzu Prominence
Flow rate: 1.0 mL/min
Mobile phase: 0.1 M Phosphate buffer 0.1 M Na2SO4, pH 6.7
Detector: UV 280 nm
Injection vol.: 20 µL
IEC-FPLC:
Column: TSKgel SP-5PW (20) (6.6 mm ID x 22 cm L, 20 µm, 1000 Å)
Flow rate: 0.85 mL/min
Buffer A: 25 mM Phosphate buffer 0.1 M Na2SO4, pH 6.0
Buffer B: A + 0.5 M NaCl
Detector: UV 280 nm
Injection vol.: 100 µL
IEC-HPLC:
Column: TSKgel SP-NPR (4.6 mm ID x 3.5 cm, 2.5 µm)
Flow rate: 1.0 mL/min
Buffer A: 25 mM Phosphate buffer 0.1 M Na2SO4, pH 6.0
Buffer B: A + 0.5 M NaCl
Detector: UV 280 nm
Injection vol.: 5 µL
RESULTS
PEGylation of lysozyme
Figure 2 shows typical chromatogram pattern of a reaction mixture of PEGylated lysozyme separated on TSKgel SP-5PW. PEG chain lengths of 5kDa and 30kDa are shown from the left to right. The profiles indicate a similar reaction characteristic. Non-reacted lysozyme remained in the reaction mixture; mono-PEGylated lysozyme as well as polyPEGylated lysozyme was formed during the reaction.
SEC was performed as shown in Figure 3. By the use of retention volumes from SEC analysis the viscosity radius of PEGylated was SEC-HPLC analysis of reaction mixes determined under assumption of being a globular protein.

FIGURE 2_Separation of PEGylated lysozymes on an analytical TSKgel SP-5PW SCX column. Peaks were identifying by MALDI-TOF analysis, identical sizes were numbered consecutively

FIGURE 3_SEC analysis of reaction mixtures performed with a TSKgel G3000SWXL column. Lysozyme and PEGylated Lysozyme derivates for all tested sizes are shown.
Selectivity
The particle size was of great importance for the selectivity. Especially the non-porous particle resin of the prepacked TSKgel SP-NPR column showed a very high resolution; with number of mono-PEGylated isoforms while two isoforms were visible for di-PEGylated lysozyme. TSKgel SP-5PW (20) is polishing resin with a particle size ten times bigger than the SP-NPR matrix. The resolution decreased, but two mono-PEGylated isoforms still remained visible (Fig. 4 A and B).

FIGURE 4_Resolution dependency on particle size shown with 5kDa PEGylated lysozyme reaction mixture. (A) TSKgel SP-NPR, (B) TSKgel SP-5PW; (1) poly-PEG5Lys, (2) 1-mono- PEG5Lys, (3) 2-mono- PEG5Lys, (4) 3-monoPEG5Lys and (5) lysozyme
DISCUSSION
Lysozyme as model protein was PEGylated to examine the behaviour of PEGylated proteins in cation exchange chromatography. A random PEGylation of lysozyme using methoxy-PEG-aldehyde of sizes 5kDa, 10 kDa and 30 kDa was performed.
In Size Exclusion Chromatography a massive increase of size by PEGylation was observed. The SEC elution behaviour of lysozyme modified with a 30 kDa PEG was equal to a 450 kDa globular protein. There was a linear correlation between the theoretical MW of PEGylated protein and the MW calculated via SEC. This result illustrates the influence of PEG on the hydrodynamic radius of PEGylated protein.
Selectivity comparison
Cation exchange chromatography was capable to resolve the PEGylated isomers which are product of the random PEGylation. The use of non-porous SP-NPR polishing resin leads to the best resolution. This is due to the better mass transfer kinetics for large molecules on small, non porous particles
Despite the loss in resolution it was useful to use a porous resin with larger particle size for the first chromatographic step because of higher capacity and better pressure-flowcharacteristics.
CONCLUSION
The selectivity of various cation exchanger resins were evaluated with random PEGylated lysozyme (chicken egg white). It is shown that the selectivity for PEG modified proteins depends on particle size of the resin. All PEGylated lysozyme species could be resolved on a TSKgel SP-NPR column with a particle size of 2.5 µm and on a TSKgel SP-5PW column with a particle size of 20 µm. A further increase of particle size leads to loss of resolution.
REFERENCE
A. Moosmann et al. J. Chromatogr. A (2010) 1217 (2): P.209-215.
