Alternate Selectivity for Polar Compounds in HILIC
Alternate Selectivity for Polar Compounds in Hydrophilic Interaction Liquid Chromatography (HILIC) Using an Amino Type HILIC Column
TOSOH
Application n°a15l63a
Hydrophilic interaction liquid chromatography (HILIC) offers unique advantages for the separation of very polar compounds when compared to reversed-phase chromatography. A new silica based HILIC phase was developed to provide additional selectivity options in HILIC separations. The separation of water soluble vitamins on the new TSKgel NH2-100 HILIC column and on the well known TSKgel Amide-80 HILIC column demonstrates the differences in selectivity.
INTRODUCTION
HILIC is used primarily to separate polar and hydrophilic compounds. Target applications for HILIC include the analysis of saccharides, glycosides, oligosaccharides, peptides and hydrophilic drugs. Altering selectivity plays a major role in maximizing resolution.
HYDROPHILIC INTERACTION CHROMATOGRAPHY
HILIC has similarities to normal phase chromatography with regard to the nature of the stationary phase. However, the eluents used for HILIC are similar to those known from reversed phase chromatography, e.g mixtures of acetonitrile and water or aqueous buffers, applied in isocratic or gradient mode. Hydrogen bonding and dipole–dipole interactions are the dominating retention mechanisms in HILIC mode. The number of polar groups, as well as the conformation and solubility of the sample in the mobile phase determine the elution order.
HILIC PHASES
Typical HILIC stationary phases are silica or polymer particles carrying polar functional groups (e.g., amino, amide or zwitterionic groups). Conventional amino type HILIC columns have limited stability in aqueous solutions but sometimes the selectivity of an amino ligand better suits the target application. Therefore, we developed a new HILIC phase, which combines the amino ligand functionality with high durability.
The new amino type HILIC phase is based on a 3 µm silica particle with 100 Å pores, which is treated with a special endcapping procedure. Amino groups are introduced step wisely after endcapping (Figure 1).
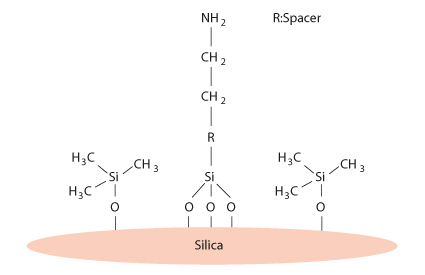
FIGURE 1_Schemiatic Diagram of the new hilic p
The amino groups act as HILIC functional groups. Because of a high ligand density and large surface area TSKgel NH2-100 3 µm columns show the strongest retention for polar compounds among the commercially available HILIC columns.
Figure 2 shows the H-u plots of the TSKgel Amide-80 and NH2-100 HILIC phases. The optimum HETP for the amino type column is reached at a flow-rate of 1.2 mL/min at a pressure of 6 MPa. At increased flow-rates the H-u-curve is relatively flat. This allows using this column for fast separations at elevated flow rates without impairing separation efficiency.
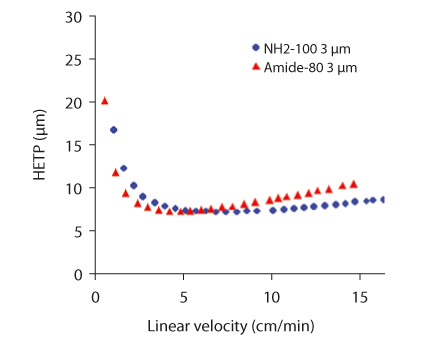
FIGURE 2_H-U-PLOTS OF TSKgel AMIDE-80 AND NH2-100 HILIC
Columns: TSKgel NH2-100 3 µm and
TSKgel Amide-80 3 µm (4.6 mm ID x 15 cm L each)
Sample: Uracil; Eluent: H2O/acetonitrile = 10/90; Flow rates: 0.1∼2.4 mL/min; Temp.: 40 °C; Detection: UV @ 254 nm

TOSOH
Application n°a15l63a
Hydrophilic interaction liquid chromatography (HILIC) offers unique advantages for the separation of very polar compounds when compared to reversed-phase chromatography. A new silica based HILIC phase was developed to provide additional selectivity options in HILIC separations. The separation of water soluble vitamins on the new TSKgel NH2-100 HILIC column and on the well known TSKgel Amide-80 HILIC column demonstrates the differences in selectivity.
INTRODUCTION
HILIC is used primarily to separate polar and hydrophilic compounds. Target applications for HILIC include the analysis of saccharides, glycosides, oligosaccharides, peptides and hydrophilic drugs. Altering selectivity plays a major role in maximizing resolution.
HYDROPHILIC INTERACTION CHROMATOGRAPHY
HILIC has similarities to normal phase chromatography with regard to the nature of the stationary phase. However, the eluents used for HILIC are similar to those known from reversed phase chromatography, e.g mixtures of acetonitrile and water or aqueous buffers, applied in isocratic or gradient mode. Hydrogen bonding and dipole–dipole interactions are the dominating retention mechanisms in HILIC mode. The number of polar groups, as well as the conformation and solubility of the sample in the mobile phase determine the elution order.
HILIC PHASES
Typical HILIC stationary phases are silica or polymer particles carrying polar functional groups (e.g., amino, amide or zwitterionic groups). Conventional amino type HILIC columns have limited stability in aqueous solutions but sometimes the selectivity of an amino ligand better suits the target application. Therefore, we developed a new HILIC phase, which combines the amino ligand functionality with high durability.
The new amino type HILIC phase is based on a 3 µm silica particle with 100 Å pores, which is treated with a special endcapping procedure. Amino groups are introduced step wisely after endcapping (Figure 1).

FIGURE 1_Schemiatic Diagram of the new hilic p
The amino groups act as HILIC functional groups. Because of a high ligand density and large surface area TSKgel NH2-100 3 µm columns show the strongest retention for polar compounds among the commercially available HILIC columns.
Figure 2 shows the H-u plots of the TSKgel Amide-80 and NH2-100 HILIC phases. The optimum HETP for the amino type column is reached at a flow-rate of 1.2 mL/min at a pressure of 6 MPa. At increased flow-rates the H-u-curve is relatively flat. This allows using this column for fast separations at elevated flow rates without impairing separation efficiency.

FIGURE 2_H-U-PLOTS OF TSKgel AMIDE-80 AND NH2-100 HILIC
Columns: TSKgel NH2-100 3 µm and
TSKgel Amide-80 3 µm (4.6 mm ID x 15 cm L each)
Sample: Uracil; Eluent: H2O/acetonitrile = 10/90; Flow rates: 0.1∼2.4 mL/min; Temp.: 40 °C; Detection: UV @ 254 nm
