HARVESTING ORGANOIDS FOR BIOCHEMICAL ANALYSIS
HARVESTING ORGANOIDS FOR BIOCHEMICAL ANALYSIS
Trevigen
HARVESTING ORGANOIDS FOR BIOCHEMICAL ANALYSIS
Sol Degese1, Gabe Benton1.
1Organoid Resource Lab (ORL), Trevigen, Inc., 8405 Helgerman Court, Gaithersburg, MD 20877
Introduction
3D Cell culture models that resemble the architecture of their tissues of origin are the next generation of medical research models. The need for a more accurate prediction of a patient’s response to a certain treatment has become key in the development of organoids. The ability to keep stem cells alive outside of the body allowed these organoids or mini-organs to be passaged, mainly in a three-dimensional matrix and never on plastic, almost indefinitely in some cases. Commonly, in order to passage or harvest these embedded organoids, proteases are employed to degrade these extracellular proteins. However, proteases also degrade proteins on the cell surface, and protease activity may carry over into subsequent cultures or lysate preparations.

Organoid Harvesting Solution, 100 ml presentation.
Catalog No. 3700-100-01
Cultrex® Organoid Harvesting Solution provides a non-enzymatic method for depolymerizing extracellular matrix proteins to allow for harvesting of intact organoids for passaging, cryopreservation, or biochemical analysis. Cultrex Organoid Harvesting Solution is compatible with the main biochemical analysis techniques, such as quantitative PCR (qPCR) and western blotting. In this report, the Organoid Resource Lab (ORL) provides you with a protocol to harvest organoids and analyze changes in gene expression by qRT-PCR or to detect your protein of interest by Western blot.
Protocol
Briefly, Mouse Small Intestine organoids (mSI) were differentiated by removing Wnt3A in the culture medium and adding DAPT, a notch inhibitor. After several days, the organoids were harvested with Cultrex Organoid Harvesting Solution. The organoid pellet was resuspended in TRIzol® (Thermo Fisher)for total RNA isolation, cDNA synthesis, and qPCR, or in RIPA buffer for preparation of total protein lysates and western blotting.
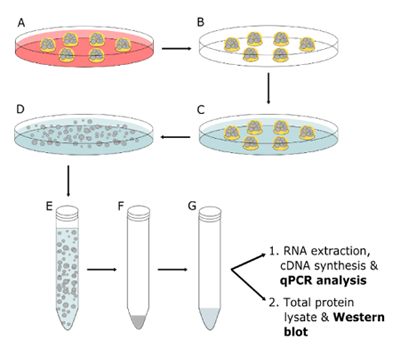
FIGURE 1_Summarized protocol to harvest organoids for biochemical analysis.
A: treat organoids with differentiation medium;
B: discard medium; C: add organoid Harvesting Solution;
D: incubate at 4°C; E: transfer organoids to a conical tube;
F: centrifuge organoids; G: resuspend organoids in the appropriate lysis solution.
Mouse Small Intestine (mSI) Organoid culture.
Organoids were grown starting from murine organoid progenitor cells from small intestine. Before the assay and at the time of passage, the cell pellet was resuspended in Cultrex BME Type R1 (catalog # 3433-005-R1) and cultured in mSI organoid culture medium for at least 3 passages. The composition of mSI organoid culture medium was the following: 50% of the final volume was completed with L Wnt3A conditioned medium, 1X B27 supplement, 1X N2 supplement, 2 mM Glutamine, 10 mM HEPES, 10 mM Nicotinamide, 1mM N-Acetylcysteine, 10 nM [Leu15]-Gastrin I Human, 1 mg/ml HA-R-Spondin1-Fc (produced using Cultrex Rspo1 cells, catalog number 3710-001-K), 100 ng/ml RecombinantMouse Noggin, 500 nM A83-01, 7.5 μg/ml Human Insulin, 10 μM SB 202190, 10 μg/ml Human Transferrin, 50 ng/ml Recombinant Mouse EGF, Advanced DMEM/F-12 Cell Culture Medium was added to complete to final volume.
Differentiation of mSI organoids. In Fig.1A, organoids were differentiated. The composition of the differentiation medium was identical to the organoid culture medium with the exception that it did not contain L Wnt3A and DAPT (a Notch inhibitor) was added fresh every medium change at a final concentration of 5 μM. The difference in volume was adjusted by adding Advanced DMEM/F12 Cell Culture Medium. For each qPCR data point, 6 domes of BME-R1 containing mSI organoids were arranged in a well of a 6-well tissue culture plate (each dome was composed by 50 μl of matrix). To detect significant morphological changes mSI organoids had to be maintained and passaged for several weeks in differentiation medium, however, to perform qPCR of enteric markers mSI organoids were maintained in differentiation medium for only 2 or 7 days.
Organoid harvesting. In Fig.1B - G the organoids were harvested and processed. In order to extract RNA and analyze gene expression, and to prepare total protein lysates, organoids were harvested using Cultrex Organoid Harvesting Solution (catalog # 3700-100-01). We recommend to harvest at least 6 domes of BME containing 100 mSI organoids or more. Culture or differentiation medium was discarded, and the wells were washed with 5 ml of cold (4°C) PBS and incubated for 30 to 60 minutes with 5 ml of cold (4°C) Cultrex Organoid Harvesting Solution. During this time, the plates were placed inside a container with ice or in a cold room with gentle shaking in order to achieve matrix depolymerization. Once the matrix was dissolved and the organoids were released, the solution was transferred to a conical centrifuge tube and centrifuged for 5 minutes at 500 x g at 4°C. The supernatant was discarded. The organoid pellet should be visible in the bottom of the tube, but it may depend on the number of organoids harvested.
RNA extraction and cDNA synthesis. The organoid pellet was resuspended in 1 ml of TRIzol. Total RNA from organoids was extracted following manufacturer’s instructions, and approximately 500 ng were used to synthesize cDNA from each sample using iScript™ cDNA synthesis kit (Bio-Rad). Alternatively, the organoid pellet can be resuspended in a different lysis buffer depending on the application.
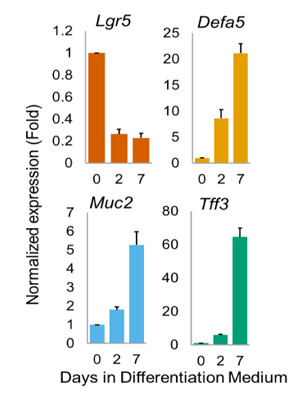
FIGURE 2_Quantitative PCR analysis.
Gene expression of the enteric markers Lgr5, Defa5,
Muc2 and Tff3 were evaluated after 0, 2 and 7 days of differentiation.
Quantitative PCR. Each cDNA sample was diluted 10 times with nuclease-free water and used as template for a quantitative PCR reaction. Each reaction had a final volume of 25 μl and contained 12.5 μl of 2X iQ™ SYBR® Green Supermix (Bio-Rad), 0.5 μl of forward and reverse qPCR primer mix 500 nM, 9.5 μl of nuclease-free water and 2.5 μl of diluted cDNA. Each sample was measured in triplicate using AB StepOnePlus™ thermocycler. All primer pairs were ordered from IDT DNA technologies. Beta-2 microglobulin was used as internal control.
Total protein lysate preparation. Organoid pellet was resuspended in 1ml of cold (4°C) RIPA buffer (50 mM Tris-HCL, pH 7.4, 1% NP-40, 0.5% Na-deoxycholate, 0.1% SDS, 150 mM NaCl, 5 mM EDTA, 50 mM NaF) plus 1X Halt™ Protease inhibitor cocktail, 1mM PMSF and 1mM Benzamidine -HCL. Working on ice, samples were vortexed and cells were lysed by either sonication of the lysates, or by passing them through a 25 gauge needle attached to a 1 ml syringe. Protein concentration was quantified and samples prepared as follows: protein concentration 0.3 μg/μl, 1X NuPage® LDS buffer and 100 mM DTT. Samples were boiled for 2 minutes and 4.5 μg of total protein was loaded in each lane.
Results
qPCR. is reduced after only 2 days of differentiation. This result is expected as the stem cell population decreases, and new crypts and villi start to form. On the other hand, the expression of Defa5, Muc2, and Tff3, all markers of intestinal differentiated cell types 1,2 was increased over time.
Western blot. Control and differentiated mSI organoids were harvested as indicated above and resuspended in RIPA buffer plus protease inhibitors. In Figure 3 a Western blot for beta II Tubulin expression shows the compatibility of Cultrex Organoid Solution with this technique.
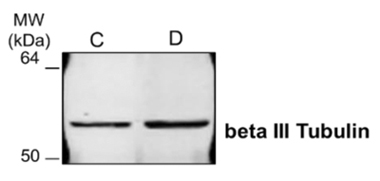
FIGURE 3_Western blot analysis.
Mouse small intestine organoid pellets, harvested with
Cultrex Organoid Harvesting Solution and resuspended in RIPA buffer.
A Western blot against beta III Tubulin for control (C)
and differentiated (D) organoids was performed.
References
1. VanDussen, K. L. et al. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut 64, 911-920, doi:10.1136/gutjnl-2013-306651 (2015).
2. Yin, X. et al. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat Methods 11, 106-112, doi:10.1038/nmeth.2737 (2014).

Trevigen
HARVESTING ORGANOIDS FOR BIOCHEMICAL ANALYSIS
Sol Degese1, Gabe Benton1.
1Organoid Resource Lab (ORL), Trevigen, Inc., 8405 Helgerman Court, Gaithersburg, MD 20877
Introduction
3D Cell culture models that resemble the architecture of their tissues of origin are the next generation of medical research models. The need for a more accurate prediction of a patient’s response to a certain treatment has become key in the development of organoids. The ability to keep stem cells alive outside of the body allowed these organoids or mini-organs to be passaged, mainly in a three-dimensional matrix and never on plastic, almost indefinitely in some cases. Commonly, in order to passage or harvest these embedded organoids, proteases are employed to degrade these extracellular proteins. However, proteases also degrade proteins on the cell surface, and protease activity may carry over into subsequent cultures or lysate preparations.

Organoid Harvesting Solution, 100 ml presentation.
Catalog No. 3700-100-01
Cultrex® Organoid Harvesting Solution provides a non-enzymatic method for depolymerizing extracellular matrix proteins to allow for harvesting of intact organoids for passaging, cryopreservation, or biochemical analysis. Cultrex Organoid Harvesting Solution is compatible with the main biochemical analysis techniques, such as quantitative PCR (qPCR) and western blotting. In this report, the Organoid Resource Lab (ORL) provides you with a protocol to harvest organoids and analyze changes in gene expression by qRT-PCR or to detect your protein of interest by Western blot.
Protocol
Briefly, Mouse Small Intestine organoids (mSI) were differentiated by removing Wnt3A in the culture medium and adding DAPT, a notch inhibitor. After several days, the organoids were harvested with Cultrex Organoid Harvesting Solution. The organoid pellet was resuspended in TRIzol® (Thermo Fisher)for total RNA isolation, cDNA synthesis, and qPCR, or in RIPA buffer for preparation of total protein lysates and western blotting.

FIGURE 1_Summarized protocol to harvest organoids for biochemical analysis.
A: treat organoids with differentiation medium;
B: discard medium; C: add organoid Harvesting Solution;
D: incubate at 4°C; E: transfer organoids to a conical tube;
F: centrifuge organoids; G: resuspend organoids in the appropriate lysis solution.
Mouse Small Intestine (mSI) Organoid culture.
Organoids were grown starting from murine organoid progenitor cells from small intestine. Before the assay and at the time of passage, the cell pellet was resuspended in Cultrex BME Type R1 (catalog # 3433-005-R1) and cultured in mSI organoid culture medium for at least 3 passages. The composition of mSI organoid culture medium was the following: 50% of the final volume was completed with L Wnt3A conditioned medium, 1X B27 supplement, 1X N2 supplement, 2 mM Glutamine, 10 mM HEPES, 10 mM Nicotinamide, 1mM N-Acetylcysteine, 10 nM [Leu15]-Gastrin I Human, 1 mg/ml HA-R-Spondin1-Fc (produced using Cultrex Rspo1 cells, catalog number 3710-001-K), 100 ng/ml RecombinantMouse Noggin, 500 nM A83-01, 7.5 μg/ml Human Insulin, 10 μM SB 202190, 10 μg/ml Human Transferrin, 50 ng/ml Recombinant Mouse EGF, Advanced DMEM/F-12 Cell Culture Medium was added to complete to final volume.
Differentiation of mSI organoids. In Fig.1A, organoids were differentiated. The composition of the differentiation medium was identical to the organoid culture medium with the exception that it did not contain L Wnt3A and DAPT (a Notch inhibitor) was added fresh every medium change at a final concentration of 5 μM. The difference in volume was adjusted by adding Advanced DMEM/F12 Cell Culture Medium. For each qPCR data point, 6 domes of BME-R1 containing mSI organoids were arranged in a well of a 6-well tissue culture plate (each dome was composed by 50 μl of matrix). To detect significant morphological changes mSI organoids had to be maintained and passaged for several weeks in differentiation medium, however, to perform qPCR of enteric markers mSI organoids were maintained in differentiation medium for only 2 or 7 days.
Organoid harvesting. In Fig.1B - G the organoids were harvested and processed. In order to extract RNA and analyze gene expression, and to prepare total protein lysates, organoids were harvested using Cultrex Organoid Harvesting Solution (catalog # 3700-100-01). We recommend to harvest at least 6 domes of BME containing 100 mSI organoids or more. Culture or differentiation medium was discarded, and the wells were washed with 5 ml of cold (4°C) PBS and incubated for 30 to 60 minutes with 5 ml of cold (4°C) Cultrex Organoid Harvesting Solution. During this time, the plates were placed inside a container with ice or in a cold room with gentle shaking in order to achieve matrix depolymerization. Once the matrix was dissolved and the organoids were released, the solution was transferred to a conical centrifuge tube and centrifuged for 5 minutes at 500 x g at 4°C. The supernatant was discarded. The organoid pellet should be visible in the bottom of the tube, but it may depend on the number of organoids harvested.
RNA extraction and cDNA synthesis. The organoid pellet was resuspended in 1 ml of TRIzol. Total RNA from organoids was extracted following manufacturer’s instructions, and approximately 500 ng were used to synthesize cDNA from each sample using iScript™ cDNA synthesis kit (Bio-Rad). Alternatively, the organoid pellet can be resuspended in a different lysis buffer depending on the application.

FIGURE 2_Quantitative PCR analysis.
Gene expression of the enteric markers Lgr5, Defa5,
Muc2 and Tff3 were evaluated after 0, 2 and 7 days of differentiation.
Quantitative PCR. Each cDNA sample was diluted 10 times with nuclease-free water and used as template for a quantitative PCR reaction. Each reaction had a final volume of 25 μl and contained 12.5 μl of 2X iQ™ SYBR® Green Supermix (Bio-Rad), 0.5 μl of forward and reverse qPCR primer mix 500 nM, 9.5 μl of nuclease-free water and 2.5 μl of diluted cDNA. Each sample was measured in triplicate using AB StepOnePlus™ thermocycler. All primer pairs were ordered from IDT DNA technologies. Beta-2 microglobulin was used as internal control.
Total protein lysate preparation. Organoid pellet was resuspended in 1ml of cold (4°C) RIPA buffer (50 mM Tris-HCL, pH 7.4, 1% NP-40, 0.5% Na-deoxycholate, 0.1% SDS, 150 mM NaCl, 5 mM EDTA, 50 mM NaF) plus 1X Halt™ Protease inhibitor cocktail, 1mM PMSF and 1mM Benzamidine -HCL. Working on ice, samples were vortexed and cells were lysed by either sonication of the lysates, or by passing them through a 25 gauge needle attached to a 1 ml syringe. Protein concentration was quantified and samples prepared as follows: protein concentration 0.3 μg/μl, 1X NuPage® LDS buffer and 100 mM DTT. Samples were boiled for 2 minutes and 4.5 μg of total protein was loaded in each lane.
Results
qPCR. is reduced after only 2 days of differentiation. This result is expected as the stem cell population decreases, and new crypts and villi start to form. On the other hand, the expression of Defa5, Muc2, and Tff3, all markers of intestinal differentiated cell types 1,2 was increased over time.
Western blot. Control and differentiated mSI organoids were harvested as indicated above and resuspended in RIPA buffer plus protease inhibitors. In Figure 3 a Western blot for beta II Tubulin expression shows the compatibility of Cultrex Organoid Solution with this technique.

FIGURE 3_Western blot analysis.
Mouse small intestine organoid pellets, harvested with
Cultrex Organoid Harvesting Solution and resuspended in RIPA buffer.
A Western blot against beta III Tubulin for control (C)
and differentiated (D) organoids was performed.
References
1. VanDussen, K. L. et al. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut 64, 911-920, doi:10.1136/gutjnl-2013-306651 (2015).
2. Yin, X. et al. Niche-independent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat Methods 11, 106-112, doi:10.1038/nmeth.2737 (2014).
