IMMUNOFLUORESCENCE OF ORGANOIDS EMBEDDED IN BASEMENT MEMBRAN
IMMUNOFLUORESCENCE OF ORGANOIDS EMBEDDED IN BASEMENT MEMBRANE MATRIX
Trevigen
Immunofluorescence of organoids embedded in Basement Membrane Matrix
Sol Degese1, Gabe Benton1
1Organoid Resource Lab (ORL), Trevigen, Inc., 8405 Helgerman Court, Gaithersburg, MD 20877
Introduction
Organoid progenitor cells are derived from embryonic, adult or induced pluripotent stem cells, and they present many similarities when compared with their tissues of origin. Basement Membrane Matrix is commonly used to provide the extracellular matrix environment required to form organoids from their respective progenitor cells. Even so, conserving intact organoids embedded in Basement Membrane Matrix to analyze the expression of markers by immunostaining represents a current challenge. Trevigen® Organoid Resource Lab (ORL) is continuously developing tools to provide the scientific community with state of the art reagents and protocols. In this instance, the ORL has developed a protocol to preserve organoids intact (and potentially other types of 3D cultures) in order to be immunostained and visualized.
Protocol
Set-up of Organoid-Basement Membrane Matrix domes. To optimize culture vessel space and to make manipulation of organoids easier, we recommend culturing organoids embedded in Cultrex® Basement Membrane Matrix BME as domes arranged in wells of a 6-well plate; this may be scaled for larger area plates if needed. Each dome contains 50 μl of resuspended organoids in matrix. In figure 1, the placement of 6 domes in each well of a 6-well plate is shown. Once finished, the plate is placed in a tissue culture incubator at 37°C to polymerize the BME. After the BME hydrogel has formed, three milliliters of organoid culture medium are added to each well.

FIGURE 1_Placement of organoid-matrix domes in wells of a 6-well plate.
1) Cultrex Basement Membrane Matrix BME and organoids are kept on ice,
while the culture vessel is pre-warmed at 37 °C to quickly induce polymerization of BME
which prevents organoids from attaching to the bottom of the well.
After harvest, the organoid pellet is resuspended in BME;
2) Domes are placed on each well by pipetting 50 μl of organoids-BME (yellow circle);
3) Six to eight domes fit in a well of a 6-well plate;
4) A 6-well plate with 6 domes in each well.
Mouse Small Intestine (mSI) Organoid culture. Organoids were grown from murine small intestine progenitor cells embedded in Cultrex BME Type R1 (catalog # 3433-005-R1) and placed in 6-well plates as shown in figure 1. Organoids were cultured in mSI organoid culture medium for at least 3 passages. The composition of mSI organoid culture medium was the following: 50% of the final volume was completed with L Wnt3A conditioned medium, 1X B27 supplement, 1X N2 supplement, 2 mM Glutamine, 10 mM HEPES, 10 mM Nicotinamide, 1mM N-Acetylcysteine, 10 nM [Leu15]-Gastrin I Human, 1 mg/ml HA-R-Spondin1-Fc (produced using Cultrex Rspo1 cells, catalog number 3710-001-K), 100 ng/ml Recombinant Mouse Noggin, 500 nM A83-01, 7.5 μg/ml Human Insulin, 10 μM SB 202190, 10 μg/ml Human Transferrin, 50 ng/ml.
Recombinant Mouse EGF, Advanced DMEM/F-12 Cell Culture Medium was added to complete to final volume. For differentiation experiments, mSI organoids were passaged and cultured in differentiation medium for several weeks. The composition of this medium was identical to mSI organoid culture medium with two exceptions: 1) it did not contain L Wnt3A, and 2) DAPT (a Notch inhibitor) was added fresh every medium change at a final concentration of 5 μM. The difference in volume was adjusted by adding Advanced DMEM/F12 Cell Culture Medium.
Mouse Colon (mCOL) Organoid culture. Organoids were grown from murine colon progenitor cells embedded in Cultrex BME Type R1 (catalog # 3433-005-R1) and placed in 6-well plates as shown in figure 1. Organoids were cultured in mCOL organoid culture medium for at least 3 passages. The composition of mCOL organoid culture medium was the following: 50% of the final volume was completed with L Wnt3A conditioned medium, 1X B27 supplement, 1X N2 supplement, 2 mM Glutamine, 10 mM HEPES, 1mM N-Acetylcysteine, 1 mg/ml HA-R-Spondin1-Fc (produced using Cultrex Rspo1 cells, catalog number 3710-001-K), 100 ng/ml Recombinant Mouse Noggin, 50 ng/ml Recombinant Mouse EGF, Advanced DMEM/F-12 Cell Culture Medium was added to complete to final volume.
Organoid harvesting and passaging. Organoids were harvested to be passaged and expanded using Cultrex Organoid Harvesting Solution (catalog # 3700-100-01). Culture or differentiation medium was discarded, and the wells were washed with 5 ml of cold (4°C) PBS and incubated for 30 to 60 minutes with 5 ml of cold (4°C) Cultrex Organoid Harvesting Solution. During this time, the plates were placed inside a container with ice or in a cold room with gentle shaking in order to achieve matrix depolymerization. Once the matrix was dissolved, and the organoids were released the solution was transferred to a conical centrifuge tube, and organoids were mechanically disrupted by passing them through a 20 gauge needle attached to a syringe. Fragmented organoids were centrifuged for 5 minutes at 500 x g at 4°C. The supernatant was discarded. The cell pellet was washed with 5-10 ml of cold (4°C) 1X PBS and centrifuged once more, and the supernatant was discarded. After a final centrifugation, PBS was discarded, and organoids were resuspended in BME.
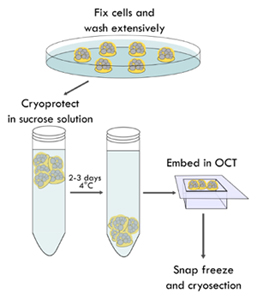
FIGURE 2_Organoids are fixed embedded in the BME domes.
Organoid domes are fixed with a PFA and GA solution, cryoprotected by immersion
in a 20% sucrose solution and embedded in OCT. After several domes were placed
in the cryomold containing OCT, the organoids are snap frozen, stored at -80°C
and sent to be cryosectioned.
Fixing of organoids, OCT embedding and cryosectioning.
Figure 2 shows a schematic representation of the following steps:
1) Remove organoid culture media, and wash each well of a 6-well plate with 5 ml of 1X PBS at room temperature.
2) Fix structures in BME with 5 ml of 2% paraformaldehyde (PFA) 0.1% glutaraldehyde (GA) in 1X PBS for 30 minutes at room temperature. Note: occasionally, PFA may cause depolymerization of the BME hydrogel, the addition of glutaraldehyde solves this issue. On the other hand, glutaraldehyde may produce higher auto-fluorescence, so it is important that the quenching step is optimized for each particular protocol.
3) Wash extensively with 5 ml of 1X PBS to remove fixing solution (at least 3 times for 10 minutes each wash).
4) Carefully, take the BME domes with a scoop or spatula and place them in a 50 ml conical tube containing 20% sucrose in 1X PBS and leave the tube at 4°C overnight or until the domes fall to the bottom of the tube (it may take up to 3 days). 5) Remove the domes from the sucrose solution and place in a mold containing Optimal Cutting Temperature (OCT) compound. Try to remove as much of the sucrose as possible. Place several domes per mold; we recommend embedding at least 6 domes of BME containing organoids in each block.
6) Snap freeze and store at -80°C.
7) Using a cryotome, cut the organoid block in cryosections of, approximately, 10 μm thick.
Immunostaining.
1) Wash the slides once with 1X PBS for 15 seconds to remove OCT.
2) Optional: use a hydrophobic marker to delimit the area around the organoids.
3) Quench aldehyde groups produced after fixing steps by incubating slides with a 10 mM solution of NaBH4 in 1X PBS twice for 5 minutes at room temperature each time. If auto-fluorescence is detected, increase the quenching washes. Note: a 15 minutes incubation done twice with 0.2 M Glycine solution in 1X PBS can be used to quench the samples instead.
4) Wash 3 times with 1X PBS for 10 minutes each time.
5) Permeabilize tissues with 0.15% Triton/1X PBS for 15 minutes at room temperature.
6) Wash 3 times with 1X PBS for 10 minutes each time.
7) Block slides with 3% BSA/1X PBS (Blocking solution) or 10% FBS/1X PBS for 1-2 hours at room temperature. Note: if a mouse-derived antibody is intended to be used with murine organoids, we suggest to use the blocking solution included in the Mouse on Mouse kits (M.O.M.™, Vector Laboratories).
8) Dilute the primary antibody in blocking solution to the desired final concentration. Tap slides on the side to remove the blocking solution and, without washing, add 200 - 400 μl of primary antibody to the organoids. Spread throughout the slide.
9) Incubate with primary antibody overnight at 4°C in a humidified chamber.
10) Wash slides twice with 1X PBS for 15 seconds.
11) Wash 3 times with 1X PBS for 10 minutes each time.
12) Dilute secondary antibodies in blocking solution to the desired final concentration. Add 200 - 400 μl of secondary antibodies to the organoids. Incubate at room temperature for 1.5 - 2 hours in a humidified chamber.
13) Wash 3 times with 1X PBS for 10 minutes each time.
14) To counterstain, use a dilution of DAPI or Hoechst 33342 for nuclear staining or Phalloidin to stain actin. All three counterstain compounds are diluted in 1X PBS.
15) Wash once with 1X PBS for 10 minutes each time.
16) Rinse in distilled water, remove as much water as possible and mount using Fluor Mounting Medium (catalog # 4866-20).
17) Wait until the slides are dry and use an epi-fluorescence or a confocal microscope to visualize your samples.
Results
Organoid lumen preservation. Both mouse small intestine and colon organoids develop a hollow lumen, as do other organoids, and it can be challenging to preserve these structures intact after fixing and cryosectioning steps. By following the protocol provided above it is possible to maintain the anatomy of organoids as seen in Figure 3, and Figure 4.
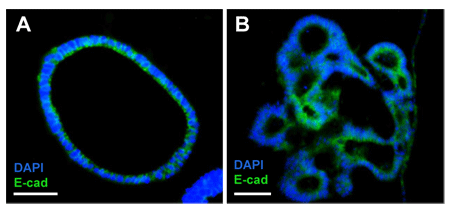
FIGURE 3_Differentiation of mouse small intestine organoids.
A) Mouse small intestine organoids usually grow as spherical hollow structures;
B) Upon differentiation, crypt- and villi-like structures start to form.
Immunofluorescence against E-cadherin (green) shows staining of tight-junctions.
Nuclei were stained with DAPI (blue). Scale bar: 50 μm.
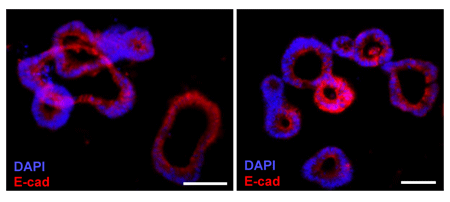
FIGURE 4_Mouse Colon Organoids.
Representative images of mouse colon organoids after 5 days in culture and immuno-stained with an anti-E-cadherin fluorescent antibody (red). Scale bar: 50 μm
Expression of intestinal markers. With this technique, it is also possible to analyze a variety of markers. In Figure 5, cells positive for Ki-67 are an indication of proliferating cells within crypt-like structures in mouse small intestine organoids. Figure 6 shows how mouse colon organoids produce and secrete mucin 2 (Muc2), a mucus forming glycoprotein secreted by Goblet cells in the intestinal epithelium.

FIGURE 5_Visualization of proliferative cells in crypt-like buds of mouse small intestine organoids.
Mouse small intestine organoids were grown embedded in Basement Membrane Matrix BME Type R1.
Ki-67 shows staining of actively proliferating cells (green, yellow arrows) in budding structures resembling of intestinal crypts (dashed white lines). DAPI was used as a nuclear counterstain (blue). Scale bar: 100 μm.
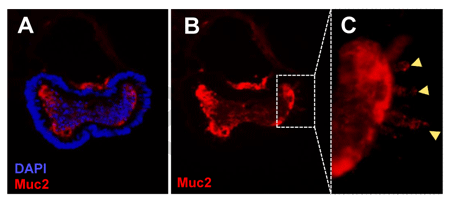
FIGURE 6_Expression of intestinal differentiation markers in mouse colon organoids.
A) Mouse Colon Organoids express the intestinal differentiation marker, Mucin 2, a Goblet cell marker (red); B) Same image as in A but showing only the red channel; C) Yellow arrows show Goblet cells expressing Mucin 2. DAPI was used as nuclear counterstain (blue).

Trevigen
Immunofluorescence of organoids embedded in Basement Membrane Matrix
Sol Degese1, Gabe Benton1
1Organoid Resource Lab (ORL), Trevigen, Inc., 8405 Helgerman Court, Gaithersburg, MD 20877
Introduction
Organoid progenitor cells are derived from embryonic, adult or induced pluripotent stem cells, and they present many similarities when compared with their tissues of origin. Basement Membrane Matrix is commonly used to provide the extracellular matrix environment required to form organoids from their respective progenitor cells. Even so, conserving intact organoids embedded in Basement Membrane Matrix to analyze the expression of markers by immunostaining represents a current challenge. Trevigen® Organoid Resource Lab (ORL) is continuously developing tools to provide the scientific community with state of the art reagents and protocols. In this instance, the ORL has developed a protocol to preserve organoids intact (and potentially other types of 3D cultures) in order to be immunostained and visualized.
Protocol
Set-up of Organoid-Basement Membrane Matrix domes. To optimize culture vessel space and to make manipulation of organoids easier, we recommend culturing organoids embedded in Cultrex® Basement Membrane Matrix BME as domes arranged in wells of a 6-well plate; this may be scaled for larger area plates if needed. Each dome contains 50 μl of resuspended organoids in matrix. In figure 1, the placement of 6 domes in each well of a 6-well plate is shown. Once finished, the plate is placed in a tissue culture incubator at 37°C to polymerize the BME. After the BME hydrogel has formed, three milliliters of organoid culture medium are added to each well.

FIGURE 1_Placement of organoid-matrix domes in wells of a 6-well plate.
1) Cultrex Basement Membrane Matrix BME and organoids are kept on ice,
while the culture vessel is pre-warmed at 37 °C to quickly induce polymerization of BME
which prevents organoids from attaching to the bottom of the well.
After harvest, the organoid pellet is resuspended in BME;
2) Domes are placed on each well by pipetting 50 μl of organoids-BME (yellow circle);
3) Six to eight domes fit in a well of a 6-well plate;
4) A 6-well plate with 6 domes in each well.
Mouse Small Intestine (mSI) Organoid culture. Organoids were grown from murine small intestine progenitor cells embedded in Cultrex BME Type R1 (catalog # 3433-005-R1) and placed in 6-well plates as shown in figure 1. Organoids were cultured in mSI organoid culture medium for at least 3 passages. The composition of mSI organoid culture medium was the following: 50% of the final volume was completed with L Wnt3A conditioned medium, 1X B27 supplement, 1X N2 supplement, 2 mM Glutamine, 10 mM HEPES, 10 mM Nicotinamide, 1mM N-Acetylcysteine, 10 nM [Leu15]-Gastrin I Human, 1 mg/ml HA-R-Spondin1-Fc (produced using Cultrex Rspo1 cells, catalog number 3710-001-K), 100 ng/ml Recombinant Mouse Noggin, 500 nM A83-01, 7.5 μg/ml Human Insulin, 10 μM SB 202190, 10 μg/ml Human Transferrin, 50 ng/ml.
Recombinant Mouse EGF, Advanced DMEM/F-12 Cell Culture Medium was added to complete to final volume. For differentiation experiments, mSI organoids were passaged and cultured in differentiation medium for several weeks. The composition of this medium was identical to mSI organoid culture medium with two exceptions: 1) it did not contain L Wnt3A, and 2) DAPT (a Notch inhibitor) was added fresh every medium change at a final concentration of 5 μM. The difference in volume was adjusted by adding Advanced DMEM/F12 Cell Culture Medium.
Mouse Colon (mCOL) Organoid culture. Organoids were grown from murine colon progenitor cells embedded in Cultrex BME Type R1 (catalog # 3433-005-R1) and placed in 6-well plates as shown in figure 1. Organoids were cultured in mCOL organoid culture medium for at least 3 passages. The composition of mCOL organoid culture medium was the following: 50% of the final volume was completed with L Wnt3A conditioned medium, 1X B27 supplement, 1X N2 supplement, 2 mM Glutamine, 10 mM HEPES, 1mM N-Acetylcysteine, 1 mg/ml HA-R-Spondin1-Fc (produced using Cultrex Rspo1 cells, catalog number 3710-001-K), 100 ng/ml Recombinant Mouse Noggin, 50 ng/ml Recombinant Mouse EGF, Advanced DMEM/F-12 Cell Culture Medium was added to complete to final volume.
Organoid harvesting and passaging. Organoids were harvested to be passaged and expanded using Cultrex Organoid Harvesting Solution (catalog # 3700-100-01). Culture or differentiation medium was discarded, and the wells were washed with 5 ml of cold (4°C) PBS and incubated for 30 to 60 minutes with 5 ml of cold (4°C) Cultrex Organoid Harvesting Solution. During this time, the plates were placed inside a container with ice or in a cold room with gentle shaking in order to achieve matrix depolymerization. Once the matrix was dissolved, and the organoids were released the solution was transferred to a conical centrifuge tube, and organoids were mechanically disrupted by passing them through a 20 gauge needle attached to a syringe. Fragmented organoids were centrifuged for 5 minutes at 500 x g at 4°C. The supernatant was discarded. The cell pellet was washed with 5-10 ml of cold (4°C) 1X PBS and centrifuged once more, and the supernatant was discarded. After a final centrifugation, PBS was discarded, and organoids were resuspended in BME.

FIGURE 2_Organoids are fixed embedded in the BME domes.
Organoid domes are fixed with a PFA and GA solution, cryoprotected by immersion
in a 20% sucrose solution and embedded in OCT. After several domes were placed
in the cryomold containing OCT, the organoids are snap frozen, stored at -80°C
and sent to be cryosectioned.
Fixing of organoids, OCT embedding and cryosectioning.
Figure 2 shows a schematic representation of the following steps:
1) Remove organoid culture media, and wash each well of a 6-well plate with 5 ml of 1X PBS at room temperature.
2) Fix structures in BME with 5 ml of 2% paraformaldehyde (PFA) 0.1% glutaraldehyde (GA) in 1X PBS for 30 minutes at room temperature. Note: occasionally, PFA may cause depolymerization of the BME hydrogel, the addition of glutaraldehyde solves this issue. On the other hand, glutaraldehyde may produce higher auto-fluorescence, so it is important that the quenching step is optimized for each particular protocol.
3) Wash extensively with 5 ml of 1X PBS to remove fixing solution (at least 3 times for 10 minutes each wash).
4) Carefully, take the BME domes with a scoop or spatula and place them in a 50 ml conical tube containing 20% sucrose in 1X PBS and leave the tube at 4°C overnight or until the domes fall to the bottom of the tube (it may take up to 3 days). 5) Remove the domes from the sucrose solution and place in a mold containing Optimal Cutting Temperature (OCT) compound. Try to remove as much of the sucrose as possible. Place several domes per mold; we recommend embedding at least 6 domes of BME containing organoids in each block.
6) Snap freeze and store at -80°C.
7) Using a cryotome, cut the organoid block in cryosections of, approximately, 10 μm thick.
Immunostaining.
1) Wash the slides once with 1X PBS for 15 seconds to remove OCT.
2) Optional: use a hydrophobic marker to delimit the area around the organoids.
3) Quench aldehyde groups produced after fixing steps by incubating slides with a 10 mM solution of NaBH4 in 1X PBS twice for 5 minutes at room temperature each time. If auto-fluorescence is detected, increase the quenching washes. Note: a 15 minutes incubation done twice with 0.2 M Glycine solution in 1X PBS can be used to quench the samples instead.
4) Wash 3 times with 1X PBS for 10 minutes each time.
5) Permeabilize tissues with 0.15% Triton/1X PBS for 15 minutes at room temperature.
6) Wash 3 times with 1X PBS for 10 minutes each time.
7) Block slides with 3% BSA/1X PBS (Blocking solution) or 10% FBS/1X PBS for 1-2 hours at room temperature. Note: if a mouse-derived antibody is intended to be used with murine organoids, we suggest to use the blocking solution included in the Mouse on Mouse kits (M.O.M.™, Vector Laboratories).
8) Dilute the primary antibody in blocking solution to the desired final concentration. Tap slides on the side to remove the blocking solution and, without washing, add 200 - 400 μl of primary antibody to the organoids. Spread throughout the slide.
9) Incubate with primary antibody overnight at 4°C in a humidified chamber.
10) Wash slides twice with 1X PBS for 15 seconds.
11) Wash 3 times with 1X PBS for 10 minutes each time.
12) Dilute secondary antibodies in blocking solution to the desired final concentration. Add 200 - 400 μl of secondary antibodies to the organoids. Incubate at room temperature for 1.5 - 2 hours in a humidified chamber.
13) Wash 3 times with 1X PBS for 10 minutes each time.
14) To counterstain, use a dilution of DAPI or Hoechst 33342 for nuclear staining or Phalloidin to stain actin. All three counterstain compounds are diluted in 1X PBS.
15) Wash once with 1X PBS for 10 minutes each time.
16) Rinse in distilled water, remove as much water as possible and mount using Fluor Mounting Medium (catalog # 4866-20).
17) Wait until the slides are dry and use an epi-fluorescence or a confocal microscope to visualize your samples.
Results
Organoid lumen preservation. Both mouse small intestine and colon organoids develop a hollow lumen, as do other organoids, and it can be challenging to preserve these structures intact after fixing and cryosectioning steps. By following the protocol provided above it is possible to maintain the anatomy of organoids as seen in Figure 3, and Figure 4.

FIGURE 3_Differentiation of mouse small intestine organoids.
A) Mouse small intestine organoids usually grow as spherical hollow structures;
B) Upon differentiation, crypt- and villi-like structures start to form.
Immunofluorescence against E-cadherin (green) shows staining of tight-junctions.
Nuclei were stained with DAPI (blue). Scale bar: 50 μm.

FIGURE 4_Mouse Colon Organoids.
Representative images of mouse colon organoids after 5 days in culture and immuno-stained with an anti-E-cadherin fluorescent antibody (red). Scale bar: 50 μm
Expression of intestinal markers. With this technique, it is also possible to analyze a variety of markers. In Figure 5, cells positive for Ki-67 are an indication of proliferating cells within crypt-like structures in mouse small intestine organoids. Figure 6 shows how mouse colon organoids produce and secrete mucin 2 (Muc2), a mucus forming glycoprotein secreted by Goblet cells in the intestinal epithelium.

FIGURE 5_Visualization of proliferative cells in crypt-like buds of mouse small intestine organoids.
Mouse small intestine organoids were grown embedded in Basement Membrane Matrix BME Type R1.
Ki-67 shows staining of actively proliferating cells (green, yellow arrows) in budding structures resembling of intestinal crypts (dashed white lines). DAPI was used as a nuclear counterstain (blue). Scale bar: 100 μm.

FIGURE 6_Expression of intestinal differentiation markers in mouse colon organoids.
A) Mouse Colon Organoids express the intestinal differentiation marker, Mucin 2, a Goblet cell marker (red); B) Same image as in A but showing only the red channel; C) Yellow arrows show Goblet cells expressing Mucin 2. DAPI was used as nuclear counterstain (blue).
