|
Solvent-free microwave-assisted synthesis of ionic liquids and their application as catalysts for benzoin condensation reaction
Audrey Aupoix, Giang Vo-Thanh* - Laboratoire de Catalyse Moléculaire, ICMMO,Université Paris-Sud 11, 91405 Orsay Cedex, FRANCE. E-mail: audrey.aupoix@icmo.u-psud.fr
Objectives: To develop eco-friendly chemical processing for the synthesis of ionic liquids using solvent-free conditions under microwave activation.To provide a highly efficient procedure for benzoin synthesis using ionic liquid both as solvent and catalyst.
Solvent-Free Microwave-Assisted Synthesis of Imidazolium and Pyridinium-based Ionic Liquids
Classical synthesis > Disadvantages : slow (2-3 days), large excess of RX, halide contamination, conventional solvent (DMF, acetonitrile, acetone,…).
Microwave synthesis > One-pot two-step microwave procedure : cleaner and greener.
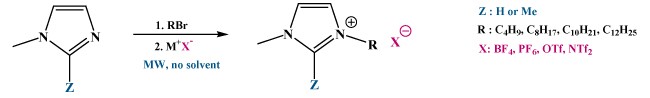
Microwave synthesis of 1-alkyl-3-methylimidazolium
8
| R |
Time (min) |
Temperature (°C) |
Yield (%) |
| N-alkylation |
Metathesis |
N-alkylation |
Metathesis |
X=OTf |
X=NTf2 |
X=PF6 |
X=BF4 |
| C4H9 |
8 |
15 |
90 |
100 |
98 |
98 |
96 |
99 |
| C8H17 |
8 |
10 |
120 |
99 |
95 |
97 |
99 |
| C10H21 |
8 |
10 |
130 |
88 |
89 |
90 |
92 |
| C12H25 |
8 |
10 |
140 |
88 |
90 |
91 |
94 |
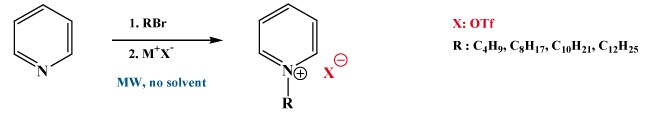
| R |
Time (min) |
Temperature (°C) |
Yield (%) |
| N-alkylation |
Metathesis |
N-alkylation |
Metathesis |
X=OTf |
| C4H9 |
10 |
10 |
130 |
120 |
99 |
| C8H17 |
10 |
10 |
130 |
130 |
91 |
| C10H21 |
10 |
10 |
140 |
120 |
96 |
Advantages > slight excess of alkyl halide, solvent-free synthesis, short reaction times (18-30 minutes).
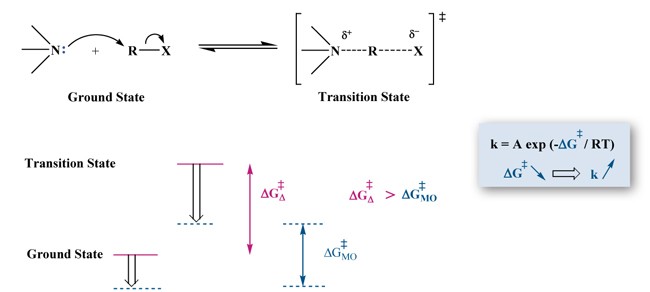
Microwave effects Specific
| Substrate |
Time (min) |
Temperature (°C) |
Yield (%) |
| N-alkylation |
Metathesis |
MW |
Δ |
| 1-Methylimidazole |
8 |
10 |
120 |
99 |
73 |
| Pyridine |
10 |
10 |
130 |
91 |
74 |
| 1,2-dimethylimidazole |
8 |
10 |
120 |
96 |
81 |
Experiments conducted under identical reaction conditions, Δ: conventional heating
R : C8H17, MX : KOTf |
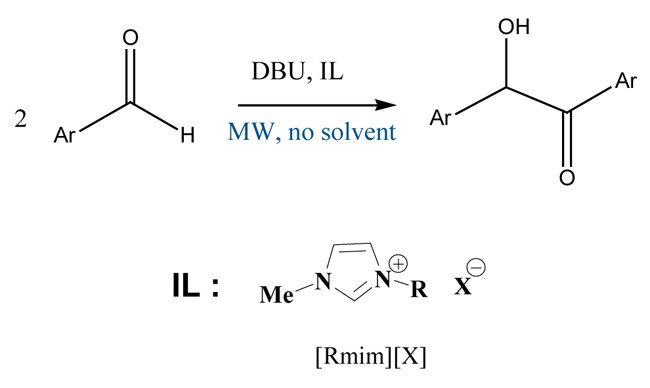
Benzoin condensation reaction
Conditions: benzaldehyde : DBU: IL (1 : 0,2 : 0,2). Temperature: 80°C. Time: 60min
Alkyl chain length effects
| Entry |
Xa |
Conversionb (%) |
Yieldb (%) |
| 1 |
OTf |
88 |
72 |
| 2 |
NTf2 |
83 |
59 |
| 3 |
BF4 |
86 |
59 |
| 4 |
PF6 |
83 |
51 |
a) IL : [Omim][X], Ar : Ph
b) Conversion and yield estimated by GC using an internal standard |
Electronic effects
| Entry |
Ar |
Conversiona (%) |
| 1 |
p-NO2-C6H4 |
0 |
| 2 |
NTf2 |
83 |
| 3 |
p-CI-C6H4 |
93 |
| 4 |
p-CH3-C6H4 |
69 |
| 5 |
p-OMe-C6H4 |
47 |
| 6 |
m-NO2-C6H4 |
0 |
| 7 |
m-CH3-C6H4 |
84 |
| 8 |
m-OMe-C6H4 |
89 |
a) Conversion estimated by NMR
IL : [Omin][OTf] ; Ar : R-Ph |
Alkyl chain length effects
| Entry |
R |
Conversiona (%) |
Yielda (%) |
| 1 |
C4H9 |
87 |
54 |
| 2 |
C8H17 |
88 |
72 |
| 3 |
C10H21 |
87 |
65 |
| 4 |
C12H25 |
85 |
59 |
a) Conversion and yield estimated by GC using an internal standard :
ethyl benzoate. (Ar : Ph) |
→ ILs : new catalysts for benzoin condensation
→ Good yields obtained in short reaction time (1 hour)
Conclusion
A rapid and highly efficient procedure for the synthesis of ionic liquids using solvent-free microwaves activation under green chemistry conditions was described.
Good to excellent yields (86-99%) were obtained in very short reaction times (18 -30 minutes). Specific microwave effects were investigated and this is in agreement with reaction mechanism. These ionic liquids are used efficiently as catalysts for benzoin condensation reaction.
|